| My turkey cooler. |
Brining Thanksgiving turkeys has been "a thing" for a while now. A quick Google search will yield dozens, if not tens of thousands, of recipes. But you only have one turkey and one chance to get it right this year, so which one should you follow?
As you might imagine, I'm going to recommend this one. For several reasons. Firstly it's quick, many recipes require you to start with a thawed bird, if you're bird hasn't been in the fridge thawing since the middle of last week it may already be too late for you to try another recipe; this one will work even if your turkey is still at the store (But if so, stop reading and go get it right now! Time is running out!). Secondly, this brine will not waste your money with ingredients that sound like a good idea but will not affect the final outcome (because science); many brine recipes call for stock or aromatics, these flavor compounds will end up down your tub drain and not in your bird. Thirdly, this recipe is very clean; the design of this recipe will keep your bird at a safe temperature with minimal effort, this combined with the high salt concentration of the water will inhibit any microbial growth. I'm not suggesting that other recipes will result in you poisoning your guests--done properly none will--but this one takes all the guess work out of keeping your bird clean and your guests safe. Finally, this recipe only makes a subtle improvement; no radical changes in flavor or texture of the bird. After you reach the end of this recipe you can season and cook your bird as is tradition in your family, the bird will taste like your family's bird only better. Sold? Then lets get too it!
I'd like to start by talking about the science of brine, but before I can do that we need some biology. Turkeys are animals (you knew that), which means they are made of lots and lots of animal cells. Animal cells look like this:
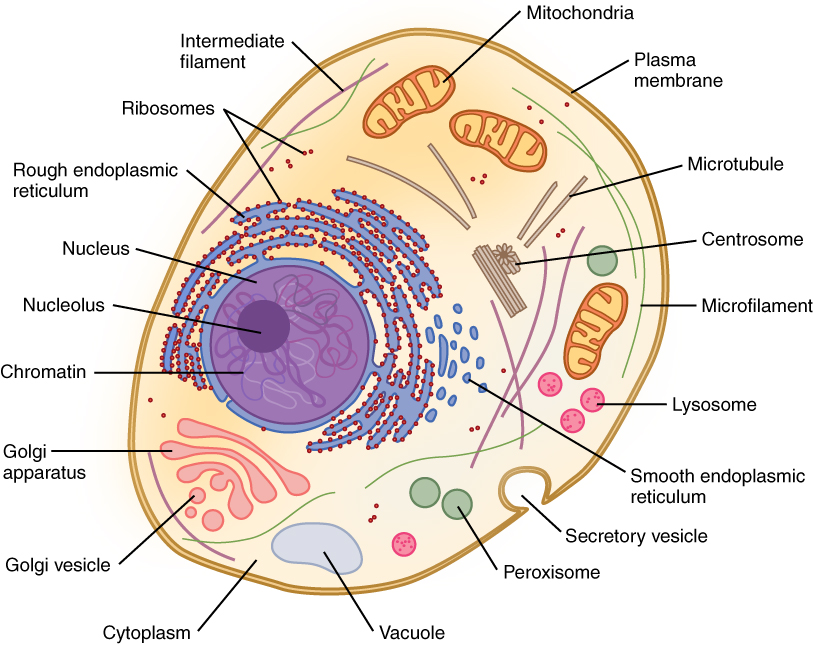 |
| Animal Cell. Image from OpenStax College via Wikimedia |
The final result is that the center of the plasma membrane is very hydrophobic and this allows the cell to keep it's insides in and the outside out. Mostly. Small molecules can sneak through via diffusion. Water mainly, in which case that's osmosis. If the cell wants anything else to get across the membrane (such as food in or waste out) it must use a transport system. These systems can be active (require energy) or passive. Once the cells are dead, only passive transport and diffusion/osmosis will continue to function.
Now back to brining. This is a very simple brine: just salt and water. The water is free to move in and out of the turkey cells via osmosis, but the water is not all that important. Even though brining makes the turkey "juicier" the water is largely irrelevant. This is because the cooking process will drive off any extra water the turkey takes on via brining, plus much of the water that was in the meat before we even started. So the magic must be in the salt. Salt (in the culinary sense) is NaCl, sodium chloride, and in water it dissolves into free Na+ and Cl- ions. Both of these ions, while small, have a very distinct charge. This means that they can not diffuse into the cells; they can't pass through the hydrophobic core of the membrane. But, there are many channels in the membrane specific for different ions. Most of these are active, but some of them work from a voltage gradient (a difference in ion concentration across the membrane) and thus are a type of semi-passive transport. While alive the cell carefully regulates the concentration of sodium, potassium, chloride, and chlorine (among others) using a combination of voltage gradient transport and active transport.
Many pixels on the internet have been spilled to say the mechanism of brine is that the chloride ions get into the meat and disrupt the structure of the muscle fibers. This prevents them from contracting during cooking, this prevents the water from being squeezed out and the meat drying out. No one talks about how the chloride gets in. Since cells spend most of their life actively pumping chloride out, without energy to keep the pump running correctly, the pump likely runs backwards and lets the chloride in. I'd like to offer an additional idea. Cells have a voltage gradient sodium calcium pump. Calcium out for sodium in. In a high sodium solution, this pump could function to remove any remaining calcium and replace it with sodium. Calcium binds to muscle fibers to allow contraction. With no calcium there can be no binding of myosin and actin, and thus no contraction. This could be working synergistically with the chloride ion's disruption. Also, of course, salt is delicious, so more salt in the meat mean more delicious meat.
This has all been a long winded way of saying, getting sodium and chloride (dissolved salt) into the cells is the goal. We can do this with a wet brine (as we will today) or by simply covering a meat in dry salt and letting it sit (maybe another time). We will be using the wet method because it allows us to take advantage of an unrelated feature of water. It's high specific heat. Specific heat is the measure of how much energy it take to raise the temperature of something. Substances with a high specific heat can absorb (or release) a lot of energy without changing temperature. In our case, cool water still has lots of energy that it can share with the frozen turkey before reaching temperature equilibrium. It can quickly thaw the bird without risk of becoming to warm. For contrast, air has a much lower specific heat, so cold (fridge) air can't donate much heat to the bird; warm air can donate more heat, thus thawing the bird more quickly, but because it is warmer, the outside of the bird will equilibrate to room temp (instead of fridge temp) and be warm enough for bacterial growth--ewww!
Brine and Thaw
1 frozen turkey 10-12lbs (I'm sure you can use a larger one. This is the size I have experience with.)
1 large cooler, big enough to hold your turkey with room for a few gallons of brine (the orange kind that are used to pour Gatorade on football coaches is great)
2 cups of salt (maybe more. buy lots)
2.5 gallons of cool water (maybe more)
A brick in a ziplock bag (squeeze out the air)
Use a knife to breech the turkey's packaging. You don't need to get it all the way off, just enough that brine can get to the bird. Put the bird in the cooler. Mix the salt and the water and add that to the cooler. Don't worry too much if some of the salt refuses to dissolve, just add it on in. If you can shove the bird down and get it completely submerged you're good. If not make more salt water of the same concentration. If you taste this you will find it to be very salty; saltier than tears, more like salty sea water. Use the brick to keep the frozen beast underwater. Seal up the cooler and take it somewhere cool. A garage is great if you have the right climate. If you're still running your AC, put the cooler in your guest bath tub. If you're worried about the turkey getting too warm you can put a digital temperature probe into the water and set an alarm for 40F, but unless you've had to put the cooler somewhere very warm (or you have a terrible cooler), the water will stay at about 33F until the bird is fully thawed. Leave the bird for 60-72 hours or so. You can check on it occasionally. Once the outside is thawed you can take off the wrapper. Once it is fully thawed, bring the bird back in the house, give it a rinse, take out the gibblets, and put it in the fridge (on a cookie sheet covered with foil) until you are ready to cook it. Ideally you will give your bird some time in the fridge to allow the skin to dry, but if you can't that's ok, just dry it with some paper towels. When you roast this turkey, be forewarned, you will often loose more liquid than you are used to; this is from the turkey absorbing water (remember, I told you the absorbed water just comes out with cooking). If you cook with the pan drippings, treat it like stock, not fat.
Now for that cooler. It's probably filled with disturbingly hot pink liquid now. Some of that is the brine used in the factory. Aren't you glad you took that out of your turkey? Some of that is just dissolved protein (same as the "blood" that often comes off of chicken you buy at the store) that leaked out due to being previously frozen. Pour that down a sink (not in your yard, all that salt will hurt your grass). Fill the cooler with hot water to at least the highest waterline of the brine. Add 1/2 cup bleach per gallon of hot water. Put the lid on and agitate the cooler to mix. Let the cooler soak for a minimum of 30min to sterilize the inside of the cooler. Pour out the bleach water and rinse well.
A note about sugar and other ingredients not included
I mentioned that I was excluding many common ingredients that don't benefit the brine. I've seen recipes that call for herbs, spices, or even stock in place of water. Flavors are made of proteins or aromatic compounds. Proteins are too big to fit through the membrane or it's channels (while alive the cells could have used endocytosis to "eat" the proteins, but the cells are dead now), and aromatic compounds are too hydrophobic to pass through the phosphate ends of the membrane. This means that all the flavors you could add at this stage will be stuck in the brine and eventually poured down the drain. Don't bother. If you want to add such flavors, add them to the bird when you prepare it for roasting.
Sugar is a different matter. Sugar will go into the cells via a channel. In fact, cells have a special channel that co-transports sodium and sugar powered by the concentration gradient of sodium across the cell membrane (cells, while alive, pump sodium out). This means adding sugar to your brine will have an effect. However, I've done it and my experience is that it gives the meat a flavor and texture that can only be described as "lunch meat". I found it very off putting; instead of juicy Thanksgiving turkey, it tasted like I was eating a slab of Oscar Myer lunch meat with gravy on it. So no sugar.

No comments:
Post a Comment